Introduction
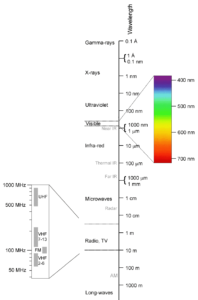
The electromagnetic spectrum is best explained with the use of an image. On the right, you see an overview of the entire range of wavelengths which, together, make up the electromagnetic spectrum which we will just call spectrum from here on.
When we look at the image we see a range of wavelengths from 0.1 Å (1 ångström = 10-10 meter = 0.1 nanometer) to 1000m. As you can see you have encountered all of these wavelengths before, be it at the doctor’s office when you broke your leg, in your kitchen when you heated up a meal, in your living room when you watched your favorite show, or in your car when you listened to the radio. When discussing color we are mainly concerned with the range of wavelengths between 400nm and 700nm: the visible or optical spectrum. It is this range of energy that humans can perceive as color. Higher energy levels than the visible part include ultraviolet, X-ray and gamma radiation. Lower energy levels include infrared, microwave, short-wave, and long-wave radiation.
Color
When we look at the visible part of the spectrum we can see that electromagnetic energy with a wavelength of 400nm represents violet light, 520nm lets us see green, 590nm yellow and 700nm results in red. When all wavelengths of the visible spectrum reach our eye simultaneously we perceive that light as being white; we do not see any specific color, just white light.
It is important to mention the terms additive and subtractive color mixing at this point. Unlike mixing pigments, which is subtractive color mixing, the transmitting of certain colors by a gemstone results in additive color mixing. Let’s take a look at the two images below.
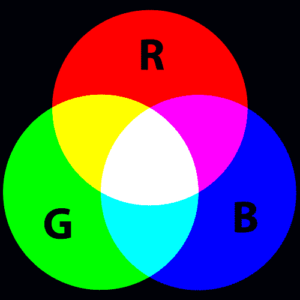
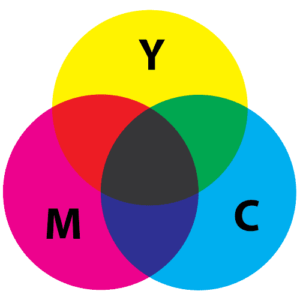
On the left you see what happens when different colors of light are mixed together: we perceive them as white. On the right you see what happens when different colors of pigment are mixed together: we perceive it to be a darker color. We all have mixed pigments at one point or another, be it at school during art class or at home painting a wall. When we mix colors the result is always darker than the component colors. This is subtractive color mixing.
The computer screen you are looking at right now is emitting three different colors per pixel: red, green and blue. The relative intensity of each of these three colors will determine which color you see. If you take a loupe or magnifying glass and hold it up to the screen you see how it works: in the white areas of your screen, the three colors are emitted in equal intensity. The text, which you perceive as black, is composed of the three colors emitting hardly anything. On the left and right, where the light green is going over into the darker green you can see a difference in the intensity of the red color pixel. In the dark green the red is clearly less bright. This additive color mixing of different colors of light is similar to what happens when you are looking at the color of a gemstone.
It is when certain wavelengths are reaching your eye in higher intensities than others that we see a certain hue. This is where gemstones get their color or lack thereof. Some colors (read: wavelengths) are being absorbed while others are transmitted. What color we see depends on the combination and intensities of the different colors that are transmitted.
Measuring Color
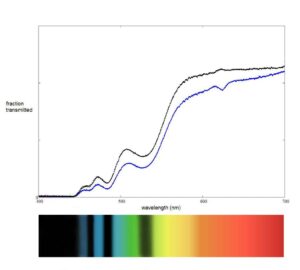
Without getting into why certain colors get absorbed while others are transmitted it is useful to note that we can register which colors are being absorbed with the aid of a spectroscope or by using a spectrometer. The spectroscope allows us to measure in a qualitative way: we see which colors are being transmitted but not how much of them is getting through, we have only a relative idea of the intensities. The spectroscope allows us to measure in a quantitative way, it will plot the intensities of the different wavelengths. Below you can see the difference: the graph is a spectrometer reading of a spessartite garnet and the spectrum below it is what can expect to see through a spectroscope.
With this information, a gemologist can identify the gemstone and, in some cases, detect certain treatments the stone may have undergone which may make dating the jewelry it is set in easier.