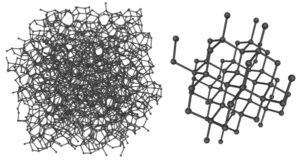
An amorphous substance is a substance that has no regular arrangement of atoms; it literally means “without form”. Examples of materials and gems that have no regular arrangement of atoms, hence no crystal structure, are glass, amber, obsidian, opal, and moldavite.
This illustration clarifies the difference between an amorphous substance and a crystalline one. On the left you can see carbon atoms which are arranged in a random way; there is no regular repeating three-dimensional array of atoms like on the right. Although both substances are composed solely of carbon atoms this arrangement of atoms makes a huge difference. The substance on the left is amorphous carbon which is very soft and brittle while the substance on the right is diamond, the hardest mineral we know.