
The earliest references to the use of platinum are to be found in Egypt at the time of the Old Kingdom. Specks of platinum group metals occur in ancient Egyptian gold work as a ‘contamination’. Because of platinum’s high density, chemical inertness and hardness, it remained with the gold throughout all purification attempts and was subsequently incorporated during the metalworking process. But these are just little specks, more substantial are the hieroglyph decorations on a copper alloy box from the 7th century BC which was excavated at Thebes. The box can be dated quite accurately because it carries the name of Shepenupet II who was an Ancient Egyptian princess of the Twenty-fifth dynasty (around 700 BC to 650 BC). A famous chemist named Marcellin Berthelot (1827-1907) reported in his 1901 work ‘Comptus Rendus'1 that he found the hieroglyphs on the back of the box to be composed of a platinum and gold alloy. Whether this was a deliberate use of platinum or a coincidence,( the maker could have mistaken a platinum-gold nugget for an electrum one) isn’t clear. Given that this appears the only use of a platinum alloy by Egyptian goldsmiths, however, it is likely that the latter is the case.

How different it was on the other side of the Atlantic, some 500 years later. Indians of the La Tolita culture, living on the border between Colombia and Ecuador near platinum-rich alluvial deposits, started to use platinum alloys in their jewelry. Quite a few artifacts such as nose rings, masks and earrings have been found to contain platinum parts, deliberately separated from other metals and crafted to form white metal objects. Quite an achievement, considering that the melting point of platinum lies at 3221.6 °F (1772.0 °C)! How did they do it?
The alluvial deposits which formed the source for platinum in the La Tolita culture mainly contain traces of the metal in the form of minute grains, although the odd bigger nugget is known to have been found. These rare, larger nuggets could theoretically be worked straight out of the river when they were of a suitable, malleable alloy. Only one such a piece is known, David Scott & Warwick Bray of the London University report:
Although native copper-iron-platinum alloys occur in Colombia they are uncommon; there is one known example from the area of a copper-platinum alloy nugget being used to make a small penannular nose-ring.2
So… that explains the origin of one object. What about the others? The Indians must have had more advanced methods to craft their platinum alloy objects. Paul Bergsoe, a Danish engineer, conducted detailed experiments on Indian platinum objects in the 1930s and shared the following:

The small grains of platinum were mixed with a little gold dust and small portions placed upon a piece of wood-charcoal. When the gold runs it will coat the grains of platinum with gold. The grains are simply “soldered” together. If the piece is now further heated by means of the blow-pipe, let us say, the following will take place: a portion of the fused gold permeates the platinum and simultaneously a little of the latter is dissolved in the molten gold. This mixture of gold and platinum can now withstand a light blow of the hammer, especially when hot. By alternately forging and heating it is possible gradually to build up an homogeneous mixture. All the specimens found are small, which is natural, since they cannot be larger if they are to be exposed to the maximum degree of heat that can be produced from a bit of charcoal and a blowpipe.3
Apart from using this technique to create a platinum alloy suitable to craft small solid objects, the goldsmiths of the La Tolita culture also used the sintered platinum alloy to create foils. The acquired thin sheets of platinum were then used to plate gold objects by hammering and heating them onto the pre-formed gold.4
European Experiments
By the end of the 17th century, the Spanish had discovered the gold deposits of present-day west Columbia and in doing so ran into the native platinum. Completely oblivious to the advances achieved by the Indians in the field of platinum alloy production, the Spanish conquistadors labeled the metal ‘little silver’ and dismissed it as a valuable metal because it wouldn’t melt with any of their known methods. Records of mines closing due to the presence of ‘platina di Pinto’ show that platinum was a nuisance to the Spanish gold miners who considered it a contamination they couldn’t get rid of.
In 1741, an Englishman named Charles Wood laid his hands on some Columbian native platinum. He passed the samples on to his brother-in-law, William Brownrigg who was a physician and scientist in England. Brownrigg conducted some experiments and finally introduced the metal to the members of the Royal Society in 1750 where it was recognized as the ‘8th metal’ (next to the traditionally known metals: Gold, Silver, Copper, Iron, Tin, Lead and Mercury).
The history of platinum continues in France, where in 1758 a Professor in Chemistry, Pierre-Joseph Macquer, had a huge burning mirror built in order to attempt the melting of platinum, an event which proved to be successful but laborious. Several attempts, with small buttons of malleable platinum as an outcome, took place over the next twenty years, almost all in or around Paris by various scientists. None of these men was able to produce workable platinum in any significant quantities though.
That honor befalls Pierre Francois Chabaneau (1754-1842), a Frenchman who, from 1781 on, was working in Spain as a French and Physics teacher at a seminary near San Sebastian. Shortly after 1783 he took the chair of Chemistry and made the production of malleable platinum his mainstay. In 1786, after just three years, he had developed a very successful method. The King of Spain ordered the secrecy of the process and gave Chabaneau a laboratory devoted to the refinery of platinum. The output of Chabaneau’s laboratory became so large that the period from 1786 until the French Invasion in 1808 is called the ‘Platinum Age in Spain’.
The process by which Chabaneau created malleable platinum is said to be a powder metallurgic one. Powdered platinum, acquired from grinding the brittle substance that was the product of early platinum purification, would be pressed, heated and hammered in order to obtain a solid, workable ingot.
Around that same time a French court jeweler, Marc Etienne Janety, was also working with platinum. In 1786 he crafted a sugar bowl out of platinum for Louis XVI. Janety used a different method than Chabaneau: the arsenic process. Platinum alloys with arsenic at low temperatures. From the molten platinum-arsenic mix a brittle, solid bar could be cast. Further heating in stages could then be applied to drive off the arsenic, producing a pure platinum bar that could be forged to shape. This method is extremely unhealthy and it’s a miracle Janety lived as long as he did.
The French Revolution (1789-1799), followed by the Napoleonic wars (1803-1815) completely disrupted scientific advances in France and Spain. As a result, the next chapter in the history of platinum takes place in England.
English Advances
Around the year 1800 two British scientists, William Hyde Wollaston and Smithson Tennant, acquainted since their days together at Cambridge University, joined forces in a quest to produce a commercially viable platinum alloy. Their research led to the discovery of the platinum group of metals. Wollaston discovered Palladium in 1802 and Rhodium in 1804. Tennant managed to identify two new members which he called Osmium and Iridium in 1803.
Wollaston produced large amounts of platinum, mainly serving the crucible and gun-producing markets. Due to its high melting point, platinum was (and still is in the case of crucibles) used to line the inside of crucibles and the touch holes of guns. The process used by Wollaston was kept secret until 1828 when he was persuaded to share his experiences with the members of the Royal Society.
His lecture revealed the following ‘recipe’:
- Precautions were taken to remove iridium as completely as possible.
- The platinum salt was decomposed at as low a temperature as possible “to occasion the particles of platina to cohere as little as possible; for on this depends the ultimate ductility of the product” and the grey product was rubbed between the hands to powder “so fine as to pass through a fine lawn sieve”.
- Great care was taken not to use anything harder than wood to break up any aggregates and not to burnish the particles, since “every degree of burnishing will prevent the particles from cohering in the further stages of the process”.
- The fine powder was washed well, removing any soluble salts, leaving a uniform pulp ready for pressing.
- The slurry was introduced under water into the die, a well-greased brass barrel about 6 inches long, tapered slightly, and closed with steel plugs.
- Pressure was applied to the plugs by a toggle press “in which the applied power was multiplied three hundred times or more”, to yield a hard cake.
- The cake was heated to redness in a charcoal fire and then placed on a layer of clean quartz, covered by a refractory pot, and sintered for twenty minutes at the maximum temperature that could be reached in a wind furnace. It was then ready for careful hot forging.5
Step one of the recipe, the complete removal of iridium, makes very little sense, after all, we know now that iridium-platinum alloys are perfectly malleable and Wollaston must have known this considering his close relation with Tennant, the discoverer of this element. Why he chose to include this piece of non-information is unclear.
Platinum Timeline
| Date | Image | Description |
|---|---|---|
| 1200 BC | The Egyptians make gold jewelry containing traces of platinum from the kingdom of Nubia. | |
| 700 BC |  | A 7th century BC Egyptian box is decorated with hieroglyphics in a gold and platinum alloy. |
| 100 BC |  | Ancient civilizations in South America used platinum for body adornment but evidence of platinum use disappears until its use is revived by the Incas circa the time of the arrival of the Spanish and Portuguese. |
| 1557 AD | First European reference to platinum found in central America at approximately Panama. | |
| 1590 AD |  | Platinum is encountered by the Spanish conquistadors in Ecuador who named it little silver, "platina". It was dismissed as an unimportant, inferior metal. |
| 18th Century | Platinum reappears during the alchemy craze as chemists and inventors use platinum as an ingredient in their formulas. | |
| 1758 AD | 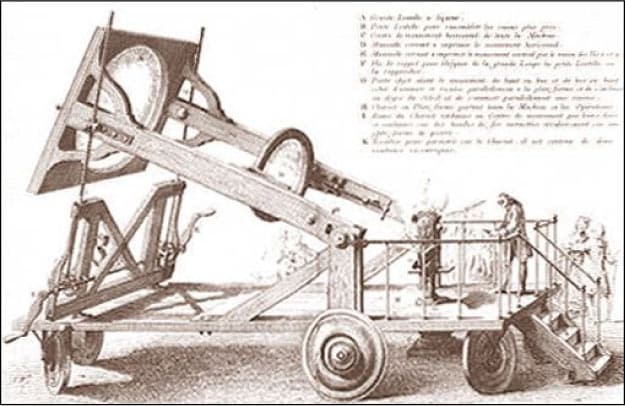 | Professor of Chemistry in the Jardin du Roi in Paris, Macquer and his assistant Antoine Baumé succeeded in 1758 in melting platinum by means of a large concave burning mirror. |
| 1786 AD |  | King Louis XVI declares platinum the only metal for kings and commissions several pieces executed in the metal. |
| 1788 AD |  | King Carlos III of Spain commissions a platinum chalice which he presents to Pope Pius VI. |
| 1795 AD |  | The metric system of weights and measures was created in France and platinum is used to create the kilogram and meter standard. |
| 1802-1804 AD | W.H.Wallaston, British scientist, discovers palladium (1802) and rhodium (1804), his colleague S. Tennant finds Osmium (1803) and Iridium (1803). These discoveries lead to the development of a more malleable platinum alloy. | |
| 1819 AD | Platinum is discovered in the Ural Mountains of Russia. | |
| 1828 AD | Russia introduces platinum coinage produced by powder metallurgy. | |
| 1857 AD | 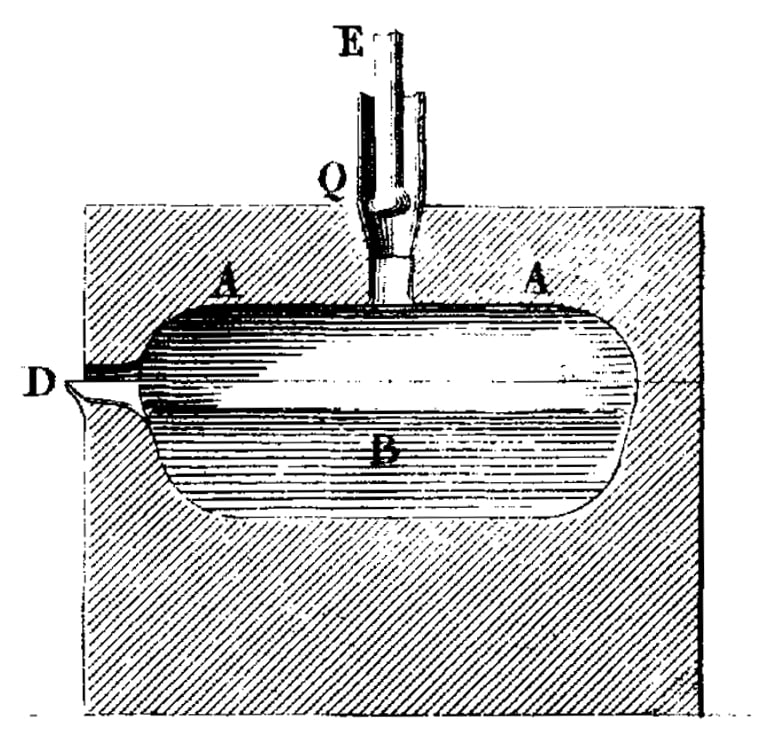 | A furnace to melt platinum and its alloys is developed by Henri Ste. Clair Deville. |
| 1912 AD | 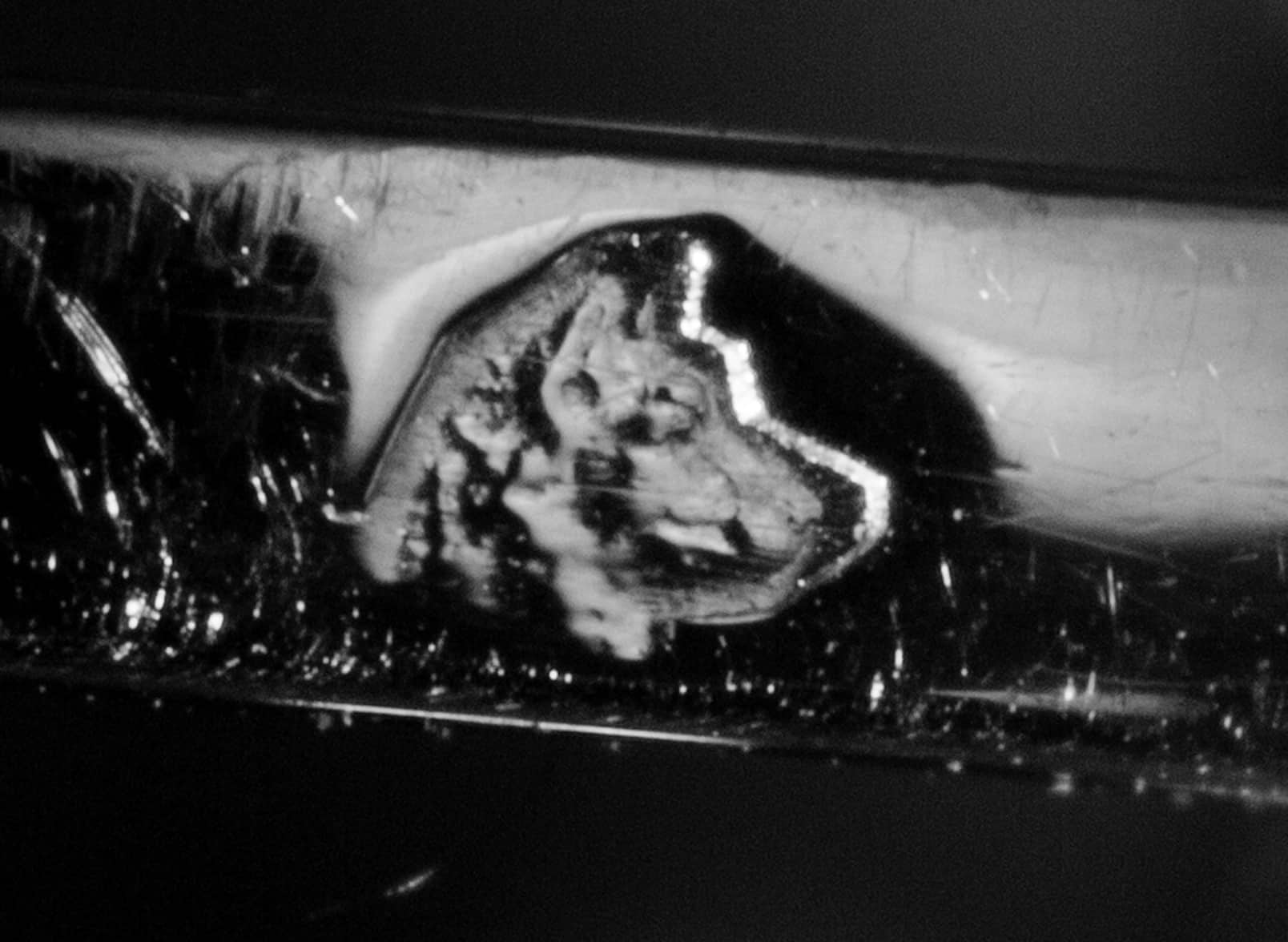 | The French start using the Dog's Head hallmark for domestic produced platinum. |
| 1924 AD |  | The world's largest platinum deposit is discovered in South Africa. |
Melting Platinum
The manufacturing of platinum by means of the powder metallurgy process was a laborious enterprise but it was the only feasible way due to the inability to melt the metal. There were simply no furnaces capable of reaching the necessary 3221.6 °F (1772.0 °C). Notable chemist Antoine Lavoisier (1743 – 1794), whose work revolved around the discovery of oxygen and hydrogen, managed to melt platinum in 1782 with a flame fueled by those two gases. His method was not suited to produce large amounts of molten platinum but the outcome of his experiments did prove that it was possible to melt the metal if high enough temperatures were reached.
The next step came a quarter of a century later, in the early 19th century, when Robert Hare from Philadelphia and English mineralogist Edward Daniel Clarke developed the oxy-hydrogen blowpipe. This tool was successful but Clarke did have to endure several explosions during his experiments. Obviously, the goldsmiths of the day weren’t too keen on adopting the blowpipe into their workshops and we do not yet see any platinum jewelry from this period.
Without a doubt inspired by Clarke and Hare, it was Henry Sainte-Claire Deville who finally developed a method to produce malleable platinum by melting purified powder. He developed a furnace with his assistant Jules Henry Debray which was powered by coal gas and pure oxygen in which high enough temperatures were reached. Accurate alloying became a possibility now, enabling Deville and Debray to produce easier-to-work alloys that couldn’t be produced by the powder metallurgy process. Deville’s furnace remained in use until the early 20th century when the electric induction furnaces took over.
The start of the widespread use of platinum in (Western) jewelry took place in the late 19th century. Around the 1870’s the first platinum appliqué work emerges. Although platinum could be melted in Deville’s furnace there was not any way for jewelers to fuse the metal to itself in their workshops. Fusing thin platinum foil to gold was possible though and consequently, this technique marked the first appearance of the metal in jewelry. A patent from 1878 mentions platinum-tipped prongs for the setting of diamonds. Around 1890 we start to see gold items that are completely topped with platinum as a precursor to solid platinum jewelry.




The invention that allowed solid platinum jewelry to be manufactured was that of liquid oxygen. Although liquid oxygen was first produced in 1877, a commercially viable process for producing didn’t come about until 1895. This enabled two French engineers, Edmond Fouché and Charles Picard to create a torch that consisted of a nozzle, a mixing chamber, and two tubes. One tube carried a fuel, such as acetylene, while the other carried pure oxygen. Oxygen and fuel mixed in the chamber and were released from the nozzle, burning at a temperature much hotter than could be reached previously. This oxyacetylene torch enabled jewelers to melt and cast platinum in their workshops and fuse it to itself. The invention of the oxyacetylene torch marked the start of solid platinum jewelry production.


Once a possibility, platinum quickly rose to be the most popular metal for jewelry purposes. Throughout the Edwardian period, its popularity was second to none. Goldsmiths could show off the metal’s strength in the incredibly delicate work so typical of this period.
Although Edward VII died in 1910, the “Edwardian” style continued until the outbreak of World War I put an abrupt end to the light-hearted Edwardian spirit. Life changed overnight and jewelry all but disappeared, either hidden away in secure vaults or sold to make ends meet. Precious metals became scarce and platinum, which was used in the manufacturing of armaments, disappeared almost entirely from the market.
Platinum jewelry returned after the War and regained its popularity in Art Deco Jewelry. This resurgence was partially made possible because of the discovery of the world’s largest platinum deposit: the Merensky Reef in northeast South Africa. It remained the metal of choice throughout the Art Deco period and into the 1940s when it was declared a strategic mineral in 1942 by the US government. Its use for jewelry then became prohibited by law in the US.
After WWII platinum jewelry took some time to regain its former status. In the sixties, it became a popular metal in Japan and in the seventies, a European revival started in Germany and spread to the rest of the continent. More recently China has become one of the principle markets for platinum jewelry.
Alloys and Purity
Pure platinum is quite soft and it is alloyed with other metals in order to increase its hardness. Most platinum alloys used for jewelry contain 85%-95% platinum. Common additives are palladium, iridium, ruthenium, cobalt or copper. What additive and ratio is used largely depends on what the goldsmith wants to do with it. An alloy of 80% platinum and 20% iridium, for instance, forms a hard and dense alloy that is extremely suitable for fine wirework. A mix of 95% platinum with 5% cobalt produces an alloy with a high viscosity when molten, a characteristic that is preferred when an object needs to be cast.
Regional preferences have formed as well. Platinum-Palladium alloys are widely used in Asia, Platinum-Cobalt is preferred in Europe and Platinum-Iridium has the US in its grip. Below follows a table that lists the most common alloys and their characteristics.6
Alloys and Purity
| Alloy | Platinum Percentage | Additive Percentage | Suitability |
|---|---|---|---|
| Pt-Palladium | 95%-85% | 5%-15% | Pt900-Pd (the 900 being parts per thousand) forms a great compromise between hardness and workability, it can be cast, welded and soldered and is favored in Japan and China. Pt850-Pd is preferred by chain makers because of its higher ductility. |
| Pt-Cobalt | 95% | 5% | This alloy is easily cast and produces hard, durable jewelry which has been favored in Europe. |
| Pt-Iridium | 95%-90% | 5%-10% | Traditionally Pt900-Ir was the alloy of choice in the US. It can be cast, welded, machined and stamped. It is ductile and malleable, can be hardened through working and doesn’t oxidize. Recently Pt950/Ir has become more popular in the States. |
| Pt-Copper | 97%-95% | 3%-5% | Platinum-copper alloys can be readily machined or worked by hand but are not as suitable for casting. |
| Pt-Ruthenium | 95% | 5% | Platinum-Ruthenium has good all-round machining properties and is well-suited to high volume manufacturing processes. It is widely used for the manufacturing of wedding bands in the US and favored by Swiss watch makers. |
Related Reading
External Link
- Ancient Egyptian materials and technology, edited by Paul T. Nicholson and Ian Shaw: http://books.google.com/books?id=Vj7A9jJrZP0C&printsec=frontcover&dq=Ancient+Egyptian+materials+and+technology&hl=en&ei=nt6aTfmBMcScOsP6zYgH&sa=X&oi=book_result&ct=result&resnum=1&ved=0CDEQ6AEwAA#v=onepage&q&f=false
- Platinum – From exotic to commodity by Wisniak, Indian Journal of Chemical Technology Vol. 12, September 2005, pp. 601-614
- Book review of ‘The Metallurgy and Technology of Gold and platinum among the Pre-Columbian Indians’ by Bergsoe et al. from the American Anthropologist volume 40, issue 1.
- The powder metallurgy of Platinum by Chaston
- The first experiments on platinum by J. Russel-Wood M.Sc., Ph.D.: http://www.platinummetalsreview.com/pdf/pmr-v5-i2-066-069.pdf
- The Platinum Chalice of Pope Pius VI By Donald McDonald: http://www.platinummetalsreview.com/pdf/pmr-v4-i2-068-069.pdf
- Ancient Platinum Technology in South America; its use by the Indians in pre-Hispanic times by Scott & Bray
- History & Etymology of Platinum: http://elements.vanderkrogt.net/element.php?sym=Pt
- Platinum Jewellery Alloys
- Henri Sainte-Claire Deville, his outstanding contributions to the chemistry and metallurgy of the platinum metals by J.C. Chaston: http://www.platinummetalsreview.com/pdf/pmr-v25-i3-121-128.pdf
- The history of melting platinum by McDonald: http://www.platinummetalsreview.com/pdf/pmr-v2-i2-055-060.pdf
- ‘A Platinum Bowl by Janety’. Clare Le Corbeiller: http://www.platinummetalsreview.com/pdf/pmr-v19-i4-154-155.pdf
Notes
- Platinum – From exotic to commodity by Wisniak, Indian Journal of Chemical Technology Vol. 12, September 2005, pp. 601-614.↵
- Ancient Platinum Technology in South America; its use by the Indians in pre-Hispanic times by Scott & Bray.↵
- Book review of ‘The Metallurgy and Technology of Gold and platinum among the Pre-Columbian Indians’ by Bergsoe et al. from the American Antropologist volume 40, issue 1.↵
- The technology of early platinum plating: a gold mask of the La Tolita culture, Ecuador.↵
- The powder metallurgy of Platinum by Chaston.↵
- Platinum jewellery Alloys.↵

